Thank you
The site provides reference information for informational purposes only. Diagnosis and treatment of diseases must be carried out under the supervision of a specialist. All drugs have contraindications. Consultation with a specialist is required!
Magnesium sulfate is a medicine that contains magnesium ions and sulfate group ions as active ingredients. Given Chemical substance has a wide range of effects on the human body. Magnesium sulfate has been used in medicine for a very long time, so all its effects are well studied and confirmed scientifically and empirically. Due to the numerous effects of magnesium sulfate, this substance is used as a symptomatic drug for a huge number of different pathological conditions.Magnesium sulfate has anticonvulsant, antiarrhythmic, vasodilator, hypotensive, antispasmodic, sedative, laxative, choleretic and tocolytic effects. That is why, when any condition occurs that magnesium sulfate can eliminate, it is used to relieve these symptoms. For example, magnesium sulfate will relieve cramps, relax the muscles of the uterus when there is a threat of miscarriage, lower blood pressure, etc.
Other names and recipe for magnesium sulfate
 Magnesium sulfate has several common names that survive from earlier times and are still in use today. So, magnesium sulfate is called:
Magnesium sulfate has several common names that survive from earlier times and are still in use today. So, magnesium sulfate is called: - Epsom salt;
- Epsom salt;
- Magnesia;
- Magnesium sulfate;
- Magnesium sulfate heptahydrate.
A prescription for magnesium sulfate is written as follows:
Rp.: Sol. Magnesii sulfatis 25% 10.0 ml
D.t. d. No. 10 in amp.
S. administer 2 ml once a day.
In the recipe, after indicating the name in Latin “Magnesii sulfatis”, write the concentration of the solution - in this example it is 25%. After which the volume is indicated, which in our example is 10 ml. After the designation "D. t. d." under the “No.” icon the number of ampoules with solution that needs to be given to a person is indicated. In this example, the number of ampoules is 10. Finally, in the last line of the recipe after the designation "S." the dosage, frequency and method of use of the drug are indicated.
Group and release forms
 Magnesium sulfate belongs to several pharmacological groups, according to the effect it has:
Magnesium sulfate belongs to several pharmacological groups, according to the effect it has:
1. Microelement;
2. Vasodilator;
3. Sedative (calming).
The medicinal substance was classified in several pharmacological groups, since magnesium sulfate has a huge number of therapeutic effects.
Today the drug is available in two dosage forms:
1.
Powder.
2.
Solution in ampoules.
The powder is available in packages of 10 g, 20 g, 25 g and 50 g. Magnesium sulfate in powder form is intended for dilution in water to obtain a suspension that can be taken orally. Magnesium sulfate solution is available in ampoules of 5 ml, 10 ml, 20 ml and 30 ml in two possible concentrations: 20% and 25%. This means that per 100 ml of solution there are, respectively, 20 g and 25 g of magnesium sulfate itself.
Magnesium sulfate powder and solution contains only this chemical. This means that there are no excipients in magnesium sulfate. That is, the drug is a simple chemical compound, which is also the active component.
Therapeutic action and pharmacological properties
Magnesium sulfate has the following therapeutic properties:- anticonvulsant;
- antiarrhythmic;
- vasodilator;
- hypotensive (reduces blood pressure);
- antispasmodic (pain reliever);
- sedative (calming);
- laxative;
- choleretic;
- tocolytic (relaxes the uterus).
Yes, when ingestion in powder form, magnesium sulfate has a choleretic and laxative effect. The choleretic effect is achieved due to irritation of receptors duodenum. And the laxative effect is due to the fact that magnesium sulfate is not absorbed into the blood, but, on the contrary, increases the flow of water into the intestinal lumen, as a result of which the stool liquefies, increases in volume, and peristaltic movements reflexively increase. As a result of this, loosening of the stool occurs.
A small portion of magnesium sulfate that is absorbed into the blood is excreted by the kidneys. That is, indirectly, magnesium has a diuretic effect. In addition, it is recommended to take magnesium sulfate orally in case of poisoning with heavy metal salts, since in such cases the chemical compound plays the role of an antidote. The drug binds heavy metals and, thanks to its laxative effect, quickly removes them from the body.
The effect of magnesium sulfate after oral administration develops within 30 minutes - 3 hours, and lasts for at least 4 - 6 hours.
Magnesium sulfate solution is used by injection and topically. Locally, the solution is used to impregnate bandages and tampons on wound surfaces. Magnesia is also used for electrophoresis, which has a beneficial effect on the central nervous system and blood vessels. In addition, electrophoresis with magnesium effectively cures warts.
Intramuscular and intravenous injections Magnesium sulfate reduces blood pressure, has a calming effect, relieves convulsions, increases urination, dilates blood vessels and eliminates cardiac arrhythmias. High doses of magnesium sulfate, administered by injection, inhibit the activity of the central nervous system, have a tocolytic, hypnotic and narcotic effect. The mechanism of action of magnesium is due to the fact that magnesium is a competitor ion to calcium. As a result, after magnesium enters the body, it competitively displaces calcium from binding sites, which reduces the amount of acetylcholine, which is the main substance regulating vascular tone, smooth muscles and nerve impulse transmission.
The anticonvulsant effect of magnesium is due to the release of acetylcholine from the neuromuscular junction and the entry of magnesium ions into it. Magnesium ions inhibit signal transmission from nerve cells to the muscles, which stops the cramps. In addition, magnesium sulfate inhibits the functioning of the central nervous system, reducing the intensity of nerve impulses, which also reduces convulsive activity. Depending on the dosage, magnesium sulfate acts on the central nervous system as a hypnotic, sedative or analgesic.
The antiarrhythmic effect of magnesium sulfate is due to a decrease in the overall ability to excite heart muscle cells, as well as normalization of the structure and functions of cardiomyocyte membranes. In addition, magnesium sulfate has a protective effect on the heart by dilating the coronary arteries and reducing the tendency of blood clots.
The tocolytic effect consists of relaxing the smooth muscles of the uterus in women and stopping their contractile activity. The muscles of the uterus relax, blood vessels dilate, contractile activity stops, as a result of which the threat of miscarriage is eliminated.
Intravenous administration Magnesium sulfate provides an almost instant effect, lasting at least half an hour. And when intramuscular injection magnesium effects develop within 1 hour and last for 3 to 4 hours.
Indications for use
Due to its numerous pharmacological and therapeutic effects, magnesium sulfate has a wide range of indications for use. In some conditions, magnesium sulfate is indicated for use in the form of injections, while in other pathologies it must be taken orally. Indications for the use of magnesium sulfate orally and by injection are shown in the table:| Indications for use of magnesium sulfate orally (powder) | Indications for the use of magnesium sulfate in the form of injections (solution) |
| Cholangitis (inflammation of the bile duct) | Hypertensive crisis, including with cerebral edema |
| Poisoning | Myocardial infarction |
| Constipation | Eclampsia in pregnancy |
| Cholecystitis | Encephalopathy |
| Colon cleansing before upcoming medical procedures | Hypomagnesemia (for example, with an unbalanced diet, taking contraceptives, diuretics, muscle relaxants, chronic alcoholism) |
| Duodenal intubation to obtain a cystic portion of bile | Increased need for magnesium (for example, during pregnancy, during adolescence, under stress, in the process of recovery) |
| Dyskinesia of the gallbladder of hypotonic type (for tubing) | As part of complex therapy for threatened miscarriage and premature birth |
| Cardiac arrhythmias | |
| Convulsions | |
| Tetany | |
| Angina pectoris | |
| Poisoning with salts of heavy metals, arsenic, tetraethyl lead, barium salts |
|
| As part of complex therapy of bronchial asthma | |
| Concussions | |
| Epileptic syndrome | |
| Urinary retention |
Magnesium sulfate (powder and solution) - instructions for use
Powder and solution have own characteristics in application, so we will consider them separately.Magnesium sulfate powder
 The powder is used internally in the form of a suspension. Before use, the required amount of powder is dissolved in warm boiled water and stirred well. The product is used regardless of food intake.
The powder is used internally in the form of a suspension. Before use, the required amount of powder is dissolved in warm boiled water and stirred well. The product is used regardless of food intake. Magnesium sulfate as cholagogue It is used as follows: dissolve 20 – 25 g of powder in 100 ml of warm boiled water. Take the resulting solution one tablespoon three times a day. To improve bile secretion, it is optimal to take magnesium sulfate before meals.
For duodenal intubation, prepare a solution as follows:
1.
10 g of powder is dissolved in 100 ml of water, obtaining a solution with a 10% concentration.
2.
12.5 g of powder is dissolved in 50 ml of water, obtaining a solution with a 25% concentration.
Then, 100 ml of a 10% or 50 ml of a 25% solution of magnesium sulfate is injected through a probe, with the help of which a bladder portion of bile is obtained. The solution administered through the probe must be warm.
An excellent remedy for this purpose is magnesium sulfate powder, or magnesia, which is a saline laxative. Magnesium sulfate acts quite gently, increasing the flow of water into the intestines, diluting stool and removing it out.
However, it should be remembered that the use of magnesium sulfate to cleanse the body is justified only before entering a diet, and not during the period of direct restriction on the quantity and quality of food consumed. You can use the drug in the first days of the diet, but not later. Magnesium sulfate will significantly facilitate the entry into therapeutic fasting, eliminating the toxins present in the body and, thereby, alleviating the unpleasant symptoms of the first days without food.
To cleanse the body before fasting or dieting for weight loss, magnesium sulfate can be used in two ways. In the first case, 30 g of powder is dissolved in half a glass of warm water and drunk before bed or any time half an hour before meals. In the second case, 30 g of powder is dissolved in half a glass of warm water and drunk in the morning, an hour after breakfast. The laxative effect develops within 4–6 hours after administration. This cleansing of the body should be carried out before entering a diet or fasting.
As an exception, you can take magnesium sulfate on the first day of a diet or fast. In this case, a person on a diet, after taking magnesium sulfate, should refrain from eating until the end of the current day. However, he will have to drink at least 2 liters of water.
Magnesium sulfate can only be used on the first day of the diet, or before entering a diet restriction regime. During a diet or fasting, you should not use magnesium sulfate to cleanse the body, as this can lead to diarrhea and dizziness, and also lead to loss of strength, vomiting, fainting, etc. Magnesium sulfate should not be used continuously, as this can lead to water-electrolyte imbalance and intestinal dysbiosis.
Magnesium sulfate for bath
Baths with magnesium sulfate have long been used as a physiotherapeutic method. A bath with magnesium will perfectly help relieve emotional and physical stress, pain, fatigue and nervousness, especially after flights, stress or anxiety. In the process of restoring balance in the body, you can take a bath with magnesium sulfate once a day, preferably before going to bed.In addition, a bath with magnesium sulfate has the following therapeutic effects:
- relieves spasm of small blood vessels;
- enhances microcirculation;
- increases uterine and renal blood flow;
- reduces blood pressure;
- reduces the formation of blood clots;
- relieves bronchospasm;
- prevents seizures in pregnant women and hypertension;
- eliminates cellulite;
- reduces muscle tone;
- enhances metabolic processes, promoting rapid recovery from injuries, fractures, serious illnesses, etc.
Tubage with magnesium sulfate
 Tubage is a procedure for cleansing the liver and gallbladder. It is optimal to carry out tubing between 18 and 20 pm. Before the procedure, you should take 1 tablet of No-shpa, and prepare a solution for tubage at the rate of 30 g of magnesium sulfate powder per 100 ml of warm boiled water. You will need 0.5 - 1 liter of this solution.
Tubage is a procedure for cleansing the liver and gallbladder. It is optimal to carry out tubing between 18 and 20 pm. Before the procedure, you should take 1 tablet of No-shpa, and prepare a solution for tubage at the rate of 30 g of magnesium sulfate powder per 100 ml of warm boiled water. You will need 0.5 - 1 liter of this solution. Then the actual tubing procedure with magnesium sulfate begins. Within 20 minutes, drink 0.5 - 1 liter of warm magnesium sulfate solution. After which the person should lie on his right side and place a heating pad on the liver area. Lie like this for 2 hours.
After tubage, a bitterness may appear in the mouth, which will go away on its own. Such tubages are made in courses of 10–16 procedures, which are carried out 1–2 times a week. Tubage should not be done in the acute stage of cholecystitis, and in the presence of erosions or ulcers in the organs gastrointestinal tract.
Magnesium sulfate for compresses
Magnesium sulfate can be used as a warm compress, which causes increased blood flow to the skin and underlying tissues. The main effects of a warm compress are pain relief and acceleration of the resorption of various seals. Warming compresses with magnesium sulfate are often applied to children at the DPT vaccination site.The compress is placed as follows:
1.
Roll the gauze into 6-8 layers.
2.
Wet gauze with a 25% solution of magnesium sulfate.
3.
Apply gauze to the injection site.
4.
Place thick paper on top for compresses.
5.
Cover the paper with cotton wool.
6.
Apply a bandage to keep the compress in place.
This compress is left for 6–8 hours, after which it is removed, the skin is washed with warm water, dried well with a towel and lubricated with a rich cream.
Magnesium sulfate
International nonproprietary name
Dosage form
Solution for injection 25%, 5 ml
Compound
5 ml of solution contain
active substance - magnesium sulfate 1.25 g,
excipient - water for injections.
Description
Transparent colorless liquid
Pharmacotherapeutic group
Plasma replacement and perfusion solutions. Additives to solutions for intravenous administration. Electrolyte solutions. Magnesium sulfate.
ATX code B05XA05
Pharmacological properties
Pharmacokinetics
The concentration of magnesium ions in blood plasma normally averages 0.84 mmol/l, 25-35% of this amount is in a protein-bound state. Penetrates well through the placenta and the blood-brain barrier; in milk it creates concentrations 2 times higher than concentrations in blood plasma. Magnesium is not metabolized.
It is excreted in the urine (at the same time increasing diuresis) by filtration; the rate of renal excretion is proportional to the concentration in the blood plasma. 93-99% of magnesium is reverse reabsorbed in the proximal and distal renal tubules.
Pharmacodynamics
When administered parenterally, it has sedative, diuretic, arteriodilating, anticonvulsant, antiarrhythmic, hypotensive, antispasmodic effects, in large doses - curare-like (depressant effect on neuromuscular transmission), tocolytic, hypnotic and narcotic effects, suppresses the respiratory center. Magnesium is a "physiological" blocker of slow calcium channels(BMKK) and is able to displace calcium from binding sites. Regulates metabolic processes, interneuronal transmission and muscle excitability, prevents the entry of calcium through the presynaptic membrane, reduces the amount of acetylcholine in the peripheral nervous system and central nervous system (CNS). Relaxes smooth muscles, reduces blood pressure (mostly elevated), increases diuresis.
Anticonvulsant action- magnesium reduces the release of acetylcholine from neuromuscular synapses, while suppressing neuromuscular transmission and has a direct inhibitory effect on the central nervous system.
Antiarrhythmic effect- magnesium reduces the excitability of cardiomyocytes, restores ionic balance, stabilizes cell membranes, disrupts sodium flow, slow incoming calcium flow and one-way potassium flow.
Hypotensive effect due to the effect of magnesium to dilate peripheral blood vessels in higher doses, in lower doses it causes sweating as a result of vasodilation.
Tocolytic action- magnesium inhibits the contractility of the myometrium (decreased absorption, binding and distribution of calcium in smooth muscle cells), increases blood flow in the uterus as a result of dilation of its vessels.
Is antidote in case of poisoning with salts of heavy metals.
Systemic effects develop almost immediately after intravenous and 1 hour after intramuscular administration. The duration of action when administered intravenously is 30 minutes, when administered intramuscularly - 3-4 hours.
Indications for use
Hypomagnesemia when it is impossible to take oral magnesium supplements
(for chronic alcoholism, severe diarrhea, malabsorption syndrome, parenteral nutrition)
Preeclampsia and eclampsia as part of complex therapy
Convulsive syndrome
Hypertensive crisis (as part of complex therapy)
Poisoning with heavy metal salts (mercury, arsenic, tetraethyl lead)
Directions for use and doses
The drug is administered intramuscularly or intravenously slowly (the first 3 ml over 3 minutes). When administered intravenously, the patient should be in a supine position.
The intravenous route of administration is considered more preferable.
Intramuscular injection is painful and can lead to the formation of infiltrates; it is used only when peripheral venous access is impossible.
The maximum dose of the drug is calculated individually based on the concentration of magnesium in the blood plasma (no more than 4 mmol/l). The duration of treatment is determined by the doctor individually depending on the clinical situation.
The dose of the drug should be reduced if renal function is impaired. Plasma magnesium concentrations should be monitored throughout the course of therapy.
Adults
Hypomagnesemia
For moderate hypomagnesemia, 4 ml of 25% (1 g) magnesium sulfate solution is administered intramuscularly every 6 hours.
For severe hypomagnesemia, the dose of the drug is 250 mg/kg body weight intramuscularly every 4 hours or 20 ml of a 25% solution of magnesium sulfate diluted per liter infusion solution(5% glucose solution or 0.9% sodium chloride solution) is administered intravenously over 3 hours.
Preeclampsia, eclampsia
In the treatment of preexlampsia and eclampsia, 5.0 g of magnesium sulfate (20 ml of 25% solution) diluted with 400 ml of 0.9% sodium chloride solution or 5% glucose is administered intravenously at a rate of 9-25 mg/min (15-40 drops). /min). As an alternative method, the Richard scheme is used: initially 4.0 g (16 ml of a 25% solution) intravenously slowly over 3-4 minutes, after 4 hours the intravenous administration is repeated at the same dose and an additional 5.0 g (20 ml 25) is administered intramuscularly % solution). Subsequently, intramuscular administration of magnesium sulfate in a dose of 4.0-5.0 g (16-20 ml of 25% solution) is repeated every 4 hours.
Continued administration of magnesium sulfate in pregnant women should not last longer than 5-7 days due to the high risk of developing congenital anomalies of the fetus.
Convulsive syndrome
For convulsive conditions, 5-10-20 ml of a 25% solution is administered intramuscularly (depending on the severity of the convulsive syndrome).
Poisoning with salts of heavy metals, mercury, arsenic
Magnesium sulfate is used as an antidote for mercury and arsenic poisoning: 5 ml of a 25% solution in a stream intravenously.
At complex treatment for chronic alcoholism, magnesium sulfate is administered intramuscularly, 5-20 ml of a 25% solution 1-2 times a day.
As part of complex therapy for hypertensive crisis
During a hypertensive crisis, 10-20 ml of a 25% solution of magnesium sulfate is administered intramuscularly or intravenously in a stream (slowly).
Elderly patients
No dose adjustment is required in elderly patients.
However, caution should be exercised if renal function is impaired.
Children
Use in children from the neonatal period, intramuscularly and intravenously.
To eliminate magnesium deficiency in newborns, magnesium sulfate is administered at a rate of 25-50 mg/kg body weight intravenously every 8-12 hours (2-3 doses).
For children to relieve seizures, the drug is prescribed at a rate of 20-40 mg/kg (0.08-0.16 ml/kg of 25% solution) intramuscularly.
When administered intravenously, magnesium sulfate is administered dropwise over 1 hour in the form of a 1% solution (10 mg/ml).
provided that half the dose is administered within the first 15-20 minutes.
Side effects
Hypersensitivity reactions
Feeling of hot flashes, sweating, diplopia
Arterial hypotension
Hypermagnesemia is characterized by hot flashes, thirst, hypotension, drowsiness, nausea, vomiting, confusion, slurred speech, double vision, loss of tendon reflexes due to neuromuscular blockade, muscle weakness, respiratory depression, electrolyte and fluid imbalance (hypophosphatemia, hyperosmolar dehydration), ECG changes(prolonged PR, QRS and QT intervals), bradycardia, cardiac arrhythmia, coma and cardiac arrest.
Depression of the respiratory center, up to paralysis of the respiratory center
Slow breathing rate, shortness of breath
Blockade of peripheral neuromuscular transmission, which leads to
weakening of tendon reflexes
Flaccid paralysis
Hypothermia
Acute circulatory failure
Anxiety, drowsiness, confusion
Polyuria
Muscle weakness, uterine atony
Hypocalcemia, with signs of secondary tetany
Contraindications
Hypersensitivity to the active substance of the drug
Severe bradycardia, atrioventricular block
Severe renal dysfunction (creatinine clearance less than 20 ml/min)
Heavy arterial hypotension
Depression of the respiratory center
Prenatal period (2 hours before birth)
Lactation period, menstruation
Drug interactions
Enhances the effect of other drugs that depress the central nervous system (tranquilizers, hypnotics).
Cardiac glycosides increase the risk of conduction disturbances and the development of atrioventricular block (especially with simultaneous intravenous administration of calcium salts).
Muscle relaxants and nifedipine enhance neuromuscular blockade.
When used together with magnesium sulfate for parenteral administration with other vasodilators, the hypotensive effect may be enhanced.
Barbiturates, narcotic analgesics, and antihypertensive drugs increase the likelihood of depression of the respiratory center.
Calcium salts reduce the effect of magnesium sulfate.
Pharmaceutically incompatible (forms a precipitate) with calcium preparations, ethanol (in high concentrations), carbonates, bicarbonates and phosphates of alkali metals, salts of arsenic acid, barium, strontium, clindamycin phosphate, hydrocortisone sodium succinate, polymyxin B sulfate, procaine hydrochloride, salicylates and tartrates .
When the concentration of magnesium ions is above 10 mmol/ml in mixtures for complete parenteral nutrition separation of fat emulsions is possible.
special instructions
Use with caution in the following conditions: myasthenia gravis, respiratory diseases, acute inflammatory diseases gastrointestinal tract.
Magnesium sulfate should be used with caution in patients with renal failure (creatinine clearance > 20 ml/min).
Parenteral use in renal failure can lead to magnesium intoxication. Patients with impaired renal function (if creatinine clearance is more than 20 ml/min) and oliguria should not receive more than 20 g of magnesium sulfate (81 mmol Mg2+) within 48 hours, and magnesium sulfate should not be administered intravenously too quickly.
Elderly patients often require a dose reduction (due to decreased renal function).
To avoid poisoning during parenteral administration of magnesium sulfate, it is necessary to carefully monitor patients and determine their level of magnesium in the blood serum.
Monitoring serum calcium levels should be routine in patients receiving magnesium sulfate.
Use in pediatrics
It is possible to use magnesium sulfate according to indications in children from the first year of life under the control of tendon reflexes and the concentration of magnesium in the blood plasma.
Pregnancy and lactation
During pregnancy, use with caution, only in cases where the expected therapeutic effect for the mother exceeds the potential risk to the fetus. It is not recommended to use the drug within 2 hours after birth.
When prescribing the drug to pregnant women, the fetal heart rate should be monitored.
Continuous administration of magnesium sulfate for 5-7 days in pregnant women can lead to hypocalcemia and bone abnormalities in the developing fetus (bone demineralization, osteopenia).
If it is necessary to use the drug during lactation, breast-feeding should be stopped.
Features of influence medicine the ability to drive vehicles and other potentially dangerous mechanisms
Overdose
Symptoms: inhibition of tendon reflexes due to neuromuscular blockade, drowsiness, confusion, slurred speech, double vision, thirst, nausea, vomiting, sharp decrease in blood pressure, bradycardia, respiratory and central nervous system depression, muscle weakness, water and electrolyte imbalance (hyperphosphatemia , hyperosmolar dehydration), ECG changes (prolongation of PR, QT and QRS complex intervals), arrhythmias, asystole.
In patients with renal failure metabolic disorders develop when prescribing lower doses.
Treatment: a 10% calcium gluconate solution of 10-20 ml is administered intravenously slowly, oxygen therapy, carbogen inhalation, artificial respiration, peritoneal dialysis or hemodialysis, and symptomatic therapy are performed.
Release form and packaging
5 ml in neutral glass ampoules or imported ones, or sterile ampoules for syringe filling.
A label made of label or writing paper is glued onto each ampoule, or the text is applied directly to the ampoule using intaglio printing ink for glass products.
5 ampoules are packed in blister packs made of polyvinyl chloride film and aluminum or imported foil.
Contour blister packs along with approved instructions for medical use in the state and Russian languages are placed in boxes made of cardboard or corrugated cardboard. The number of instructions is nested according to the number of packages.
Storage conditions
In a place protected from light at a temperature not exceeding 30°C.
Keep out of the reach of children.
Shelf life
After the expiration date, do not use the drug.
Conditions for dispensing from pharmacies
On prescription
Manufacturer
JSC "Khimpharm", Republic of Kazakhstan,
Shymkent, st. Rashidova, w/n, t/f: 560882
Registration Certificate Holder
JSC "Khimpharm", Republic of Kazakhstan
Address of the hosting organization on the territory of the Republic of Kazakhstan complaints from consumers regarding product (product) quality
JSC "Khimpharm", Shymkent, REPUBLIC OF KAZAKHSTAN,
st. Rashidova, w/n, t/f: 560882
Phone number 7252 (561342)
Fax number 7252 (561342)
E-mail address [email protected]
APPROVED
By order of the chairman
Committee for Control of Medical and
Pharmaceutical activities
Ministry of Health
Republic of Kazakhstan
From "____"______________201__
№ ________________
Instructions for medical use
Medicine
MAGNESIUM SULPHATE-DARNITSA
Magnesium sulfate - Darnitsa
International generic name
Solution for injection 25% 5 ml, 10 ml
Compound
1 ml of solution contains
active substance- magnesium sulfate 250 mg,
excipient- water for injections.
Description
Transparent colorless liquid.
Pharmacotherapeutic group
Plasma replacement and perfusion solutions. Additives to solutions for intravenous administration. Electrolyte solutions. Magnesium sulfate.
Code ATX В05ХА05
Pharmacological properties
Pharmacokinetics
Passes through the blood-brain barrier and placenta, excreted into breast milk, the concentration of which is 2 times higher than the concentration in blood plasma. Excreted by the kidneys, the rate of renal excretion is proportional to plasma concentration and level glomerular filtration. The plasma concentration at which the anticonvulsant effect develops is 2-3.5 mmol/l.
Pharmacodynamics
When administered parenterally, it has a hypotensive, arteriolodilating, antiarrhythmic, sedative, anticonvulsant, diuretic, antispasmodic, and tocolytic effect. Replenishes magnesium deficiency in the body and is a physiological calcium antagonist. Regulates metabolic processes, neurochemical transmission and muscle excitability, prevents the entry of calcium ions through the presynaptic membrane, reduces the amount of acetylcholine in the peripheral and central nervous system, has sedative, hypnotic or narcotic effects depending on the dose, and has an antispasmodic effect. Reduces the excitability of the respiratory center; when administered in high doses, it can cause respiratory depression.
The hypotensive and antiarrhythmic effects of magnesium are due to a decrease in the excitability of cardiomyocytes, restoration of ionic balance, stabilization of cell membranes, disruption of sodium flow, slow incoming calcium flow and one-way potassium flow, dilation of the coronary arteries, reduction of total peripheral vascular resistance, platelet aggregation, as well as antispasmodic and sedative effects.
The sedative and anticonvulsant effects of magnesium are associated with a decrease in the release of acetylcholine from neuromuscular synapses, inhibition of neuromuscular transmission, and a direct inhibitory effect on the central nervous system.
The tocolytic effect develops due to inhibition of the ability to contract the myometrium (decreased absorption, binding and distribution of calcium in smooth muscle cells), vasodilation and increased blood flow in the uterus. Magnesium has antispasmodic effect with urinary retention, is an antidote for poisoning with heavy metal salts.
Systemic effects develop almost instantly after intravenous and 1 hour after intramuscular administration, their duration is 30 minutes and 3-4 hours, respectively.
Indications for use
Hypertensive crisis, ventricular cardiac arrhythmias (pirouette-type tachycardia)
Convulsive syndrome
Eclampsia
Hypomagnesemia, increased need for magnesium
In case of poisoning with salts of heavy metals, tetraethyl lead, soluble barium salts (antidote) in complex therapy
Method of administration and dose.
Prescribed intramuscularly, intravenously slowly or as an intravenous infusion. Freshly prepared infusion solutions cannot be stored for long periods of time and should be used immediately after preparation. The frequency of administration and dose are individual depending on the indications and therapeutic effect. For infusion administration, the drug is diluted with 0.9% sodium chloride solution or 5% glucose. For intravenous injection, the rate of administration should usually not exceed 150 mg/min (0.6 ml/min), with the exception of the treatment of arrhythmias and eclampsia of pregnancy.
Hypomagnesemia. For moderately severe hypomagnesemia (0.5-0.7 mmol/l), adults are administered 4 ml (1 g of magnesium sulfate) intramuscularly every 6 hours.
For severe hypomagnesemia (< 0,5 ммоль/л) при внутримышечном введении суммарную дозу повышают до 1 мл/кг (250 мг/кг) и вводят частями в течение 4 часов. В виде внутривенной инфузии при тяжелой гипомагниемии 20 мл препарата (5 г магния сульфата) добавляют к 1 л 0,9 % раствора натрия хлорида или 5 % глюкозы и вводят в течение не менее 3 часов.
Maximum daily dose when administered intravenously, it is 72 ml (18 g). If necessary, infusions are repeated over several days.
Arterial hypertension. At arterial hypertension Stages I-II are administered intramuscularly daily at 5-10-20 ml. The course of treatment is 15-20 injections, while, along with a decrease in blood pressure, a decrease in the severity of angina pectoris may be observed.
Hypertensive crisis. Inject 10-20 ml intramuscularly or intravenously in a stream, slowly.
Cardiac arrhythmias. To relieve arrhythmias, 4-8 ml (1-2 g of magnesium sulfate) are administered intravenously over 5-10 minutes, repeat the injection if necessary (total administration of up to 4 g of magnesium sulfate).
It is possible to administer first a loading dose of 8 ml for at least 5 minutes, followed by an infusion of 20 ml of the drug diluted with a solution of 0.9% sodium chloride or 5% glucose for at least 6 hours, or first 8 ml for at least 30 minutes followed by infusion over at least 12 hours.
Convulsive syndrome. Adults: 5-10-20 ml intramuscularly. Children are administered intramuscularly at a rate of 0.08-0.16 ml/kg (20-40 mg/kg).
For eclampsia. 10-20 ml 1-2 times a day intramuscularly (can be combined with simultaneous administration neuroleptics).
For preeclampsia or eclampsia, it is administered intramuscularly or intravenously. First, 10 ml is injected once into each buttock or 16 ml (4 g of magnesium sulfate) intravenously over 3-4 minutes. Then continue to administer intramuscularly 16-20 ml (4-5 g) every 4 hours or intravenously drip 4-8 ml/hour (1-2 g/hour) with constant monitoring of tendon reflexes and respiratory function. Therapy is continued until the attack stops. The maximum daily dose is 40 g of magnesium sulfate, in case of impaired renal function - 20 g/48 hours.
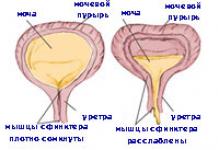
Urinary retention. For urinary retention and lead colic, 5-10 ml of the drug is administered intramuscularly or 5-10 ml of a 25% solution of magnesium sulfate diluted 5 times (also prescribed as an enema).
Like an antidote. In case of intoxication with mercury, arsenic, tetraethyl lead, 5-10 ml of a 25% solution of magnesium sulfate diluted 2.5-5 times is administered intravenously. In case of poisoning with soluble barium salts, 4-8 ml are administered intravenously or the stomach is washed with a 1% solution of magnesium sulfate.
Newborns. At intracranial hypertension and severe asphyxia in newborns is administered intramuscularly, starting with a dose of 0.2 ml/kg/day, increasing the dose on the 3-4th day to 0.8 ml/kg/day for 3-8 days in a complex therapy. To eliminate magnesium deficiency in newborns, 0.5-0.8 ml/kg is prescribed once a day for 5-8 days.
Arterial hypotension, bradycardia, palpitations, conduction disturbances, hot flashes, prolongation of the PQ interval and expansion of the QRS complex on the ECG, arrhythmia, coma, cardiac arrest
Dyspnea, respiratory depression
Headache, dizziness, general weakness, drowsiness, confusion, loss of consciousness, depressed mood, decreased tendon reflexes, diplopia, anxiety, speech disorders, tremors and numbness of the extremities
Muscle weakness
Nausea, vomiting, diarrhea
Anaphylactic shock, angioedema, hyperthermic syndrome, chills
Hyperemia, itching, rashes, urticaria, increased sweating
Polyuria
Uterine atony
Hypocalcemia, hypophosphatemia, hyperosmolar dehydration
Contraindications
Increased individual sensitivity to the components of the drug
Arterial hypotension, severe bradycardia (heart rate less than 55 beats/min), atrioventricular block
Conditions caused by calcium deficiency and depression of the respiratory center, severe respiratory diseases
Cachexia
Impaired renal function, severe hepatic or renal failure
Myasthenia gravis
Prenatal period (2 hours before birth), lactation period
Menstruation
Use with caution for myasthenia gravis, respiratory diseases, acute inflammatory diseases of the gastrointestinal tract, and pregnancy.
Calcium ions have an antagonistic effect towards magnesium ions, which, when used simultaneously, leads to a decrease in the pharmacological effects of magnesium sulfate. Enhances the effect of drugs that depress the central nervous system (narcotics, analgesics). With the simultaneous use of muscle relaxants and nifedipine, neuromuscular blockade increases. Simultaneous use with calcium channel blockers such as nifedipine can lead to calcium imbalance and impaired muscle function. Barbiturates, narcotic analgesics and antihypertensive drugs increase the likelihood of depression of the respiratory center.
Cardiac glycosides increase the risk of developing conduction disorders and atrioventricular block.
The effect of antithrombotic agents, vitamin K antagonists, isoniazid, and non-selective inhibitors of neuronal monoamine reuptake is reduced.
The elimination of mexiletine may be slower. Dosages may need to be revised.
Propafenone - the effect of both drugs is enhanced and the risk of toxic effects increases.
It interferes with the absorption of tetracycline antibiotics, intestinal obstruction is possible, and weakens the effect of streptomycin and tobramycin.
Pharmaceutically incompatible (precipitates form) with calcium preparations, ethanol (in high concentrations), carbonates, bicarbonates and phosphates of alkali metals, arsenic acid, barium, strontium salts, clindamycin phosphate, hydrocortisone sodium succinate, polymyxin B sulfate, procaine hydrochloride, salicylates and tartrates . At Mg2+ concentrations above 10 mmol/ml in total parenteral nutrition formulas, distribution of fat emulsions is possible.
Before starting therapy, the level of magnesium in the blood should be determined. In adults normal level magnesium in blood plasma is 0.75-1.26 mmol/l.
When using the drug, it should be taken into account that an increase in the excretion of magnesium in the urine occurs with an increase in extracellular fluid, dilation of the renal vessels, hypercalcemia, increased excretion of sodium in the urine, when prescribing osmotic diuretics (urea, mannitol, glucose), “loop” diuretics (furosemide, ethacrine acid, thiazides), when taking cardiac glycosides, calcitonin, thyroidin, with long-term administration of deoxycorticosterone acetate (more than 3-4 days). A slowdown in magnesium excretion is observed with the administration of parathyroid hormone. In case of renal failure, the excretion of magnesium slows down, and with repeated administrations, its accumulation may occur. Therefore, in elderly patients and in patients with severe renal impairment, the dose of the drug should not be more than 20 g of magnesium sulfate (81 mmol Mg2+) within 48 hours; patients with oliguria or severe renal impairment should not administer magnesium sulfate intravenously quickly. Urinary tract infections accelerate the precipitation of ammonium magnesium phosphates, and magnesium therapy is temporarily not recommended. If the excretion of magnesium is impaired after parenteral administration of magnesium sulfate, hypermagnesemia is possible.
Use with caution for myasthenia gravis and respiratory diseases. With long-term use of the drug, monitoring is recommended of cardio-vascular system, tendon reflexes, kidney function and respiratory rate.
Intravenous administration of magnesium sulfate is carried out slowly: if the rate of administration is too high, hypermagnesemia is possible (symptoms are nausea, paresthesia, sedation, hypoventilation up to apnea, decreased deep tendon reflexes). Simultaneous parenteral administration of vitamin B6 and insulin increases the effectiveness of magnesium therapy.
If it is necessary to simultaneously administer magnesium sulfate and calcium preparations, they should be injected into different veins, and it must be taken into account that the level of magnesium depends on the level of calcium in the body.
The drug is used in pediatric practice.
Pregnancy, lactation period
Magnesium sulfate penetrates the placenta; long-term therapy (more than 3 weeks) promotes leaching of calcium from the fetus.
During pregnancy, magnesium sulfate is used with caution, taking into account the concentration of magnesium in the blood, in cases where the expected therapeutic effect outweighs the potential risk to the fetus. When anesthetizing labor, one should take into account the possibility of inhibition of the contractility of the uterine muscles, which requires the use of birth stimulants.
Instructions for use
Magnesium sulfate instructions for use
Dosage form
Transparent colorless liquid.
Compound
Composition of the drug per 1 ml:
Active substance:
Magnesium sulfate heptahydrate - 250 mg.
Excipients:
1 M sodium hydroxide solution - up to pH 5.5 - 0.8
Water for injections - up to 1 ml.
Pharmacodynamics
When administered parenterally, it has an anticonvulsant, antiarrhythmic, antihypertensive, antispasmodic effect; in large doses, it inhibits neuromuscular transmission, has a tocolytic effect, and suppresses the respiratory center.
Magnesium is a physiological antagonist of calcium and is able to displace it from binding sites. Regulates metabolic processes, interneuronal transmission and muscle excitability, prevents the entry of calcium ions through the presynaptic membrane, reduces the amount of acetylcholine in the peripheral nervous system and central nervous system. Relaxes smooth muscles, reduces blood pressure (mostly elevated), increases diuresis. The mechanism of anticonvulsant action is associated with a decrease in the release of acetylcholine from neuromuscular synapses, while magnesium suppresses neuromuscular transmission and has a direct inhibitory effect on the central nervous system.
The antiarrhythmic effect of magnesium is due to a decrease in the excitability of cardiomyocytes, restoration of ionic balance, stabilization of cell membranes, disruption of sodium current, slow incoming current of calcium ions and one-way potassium current.
The gocolytic effect develops as a result of inhibition of myometrial contractility (decreased absorption, binding and distribution of calcium ions in smooth muscle cells) under the influence of magnesium ions, increased blood flow in the uterus as a result of dilation of its vessels.
Magnesium is an antidote for poisoning with heavy metal salts.
Systemic effects develop almost immediately after intravenous administration. Duration of action when administered intravenously is 30 minutes.
Pharmacokinetics
The equilibrium concentration of Css is 2-3.5 mmol/l. Penetrates the blood-brain barrier and the placental barrier, creating concentrations in breast milk that are 2 times higher than those in plasma. Excretion is carried out by the kidneys, its rate is proportional to plasma concentration and the level of glomerular filtration.
Side effects
Early signs and symptoms of hypermagnesemia: bradycardia, diplopia, sudden “rush” of blood to the facial skin, headache, decreased blood pressure, nausea, shortness of breath, slurred speech, vomiting, asthenia.
Signs of hypermagnesemia, ranked in order of increasing the content of magnesium ions in the blood serum: decreased deep tendon reflexes (2-3.5 mmol/l), prolongation of the PQ interval and expansion of the QR.S complex on the electrocardiogram (2.5-5 mmol/l) , loss of deep tendon reflexes (4-5 mmol/l), depression of the respiratory center (5-6.5 mmol/l), cardiac conduction disturbance (7.5 mmol/l), cardiac arrest (12.5 mmol/l) .
Hyperhidrosis, anxiety, deep sedation, polyuria, uterine atony.
The drug reduces the excitability of the respiratory center, large doses When administered parenterally, the drug can cause paralysis of the respiratory center.
Selling Features
prescription
Special conditions
If simultaneous intravenous administration of magnesium and calcium salts is necessary, they are injected into different veins.
It is possible to use magnesium sulfate to relieve status epilepticus (as part of complex therapy).
Patients with severe renal impairment (creatinine clearance more than 20 ml/min) should not receive more than 20 g of magnesium sulfate (81 mmol Mg2*) per 48 hours; patients with oliguria or severe renal impairment should not administer magnesium sulfate intravenously too quickly. It is recommended to monitor the content of magnesium ions in the blood serum (should not be higher than 0.8-1.2 mmol/l), diuresis (at least 100 ml/h), respiratory rate (at least 16/min), blood pressure, monitoring is necessary tendon reflexes.
When used parenterally, special care should be taken to avoid creating toxic concentrations of the drug. Elderly patients often require dose reduction (weakening of kidney function).
When using magnesium sulfate, the results of radiological studies for which technetium is used may be distorted.
Effect of the drug on the ability to drive vehicles and other mechanisms:
During the treatment period, care must be taken when driving vehicles and engaging in other potentially dangerous species activities that require increased concentration and speed of psychomotor reactions.
Indications
Arterial hypertension (including hypertensive crisis with symptoms of cerebral edema), polymorphic ventricular tachycardia (pirouette type), convulsive syndrome(to suppress seizures in eclampsia, to prevent seizures in severe preeclampsia, to relieve strong uterine contractions), poisoning with heavy metal salts (mercury, arsenic, tetraethyl lead), hypomagnesemia (including increased need for magnesium and acute hypomagnesemia).
Contraindications
Hypersensitivity to the components of the drug, severe arterial hypotension, depression of the respiratory center, severe bradycardia, atrioventricular block (AV block I-III degree), severe chronic renal failure (creatinine clearance less than 20 ml/min), prenatal period (2 hours before birth ), conditions associated with calcium deficiency.
Carefully:
Myasthenia gravis, chronic renal failure (if creatinine clearance is more than 20 ml/min), respiratory diseases, acute inflammatory diseases of the gastrointestinal tract, pregnancy, elderly age, lactation period, age up to 18 years.
Pregnancy and lactation:
The use of the drug during pregnancy and lactation is possible only as prescribed by a doctor, if the expected benefit to the mother outweighs the potential risk to the fetus or child.
If it is necessary to use the drug during lactation, breastfeeding should be stopped.
Drug interactions
Enhances the effect of other drugs that depress the central nervous system.
Cardiac glycosides increase the risk of conduction disturbances and atrioventricular (AV) block (especially with simultaneous intravenous administration of calcium salts).
Muscle relaxants and nifedipine enhance neuromuscular blockade.
When combined with magnesium sulfate for parenteral administration with other vasodilators, the hypotensive effect may be enhanced.
Barbiturates, narcotic analgesics, and antihypertensive drugs increase the likelihood of depression of the respiratory center.
It interferes with the absorption of tetracycline antibiotics and weakens the effect of streptomycin and tobramycin.
Calcium salts reduce the effect of magnesium sulfate.
Pharmaceutically incompatible (forms a precipitate) with calcium preparations, ethanol (in high concentrations), carbonates, bicarbonates and phosphates of alkali metals, salts of arsenic acid, barium, strontium, clindamycin phosphate, hydrocartisone sodium succinate, polymyxin B sulfate, procaine hydrochloride,
Salicylates and tarrates. When the content of magnesium ions is above 10 mmol/ml in mixtures for total parenteral nutrition, separation of fat emulsions is possible.
Prices for Magnesium sulfate in other cities
Buy Magnesium sulfate,Magnesium sulfate in St. Petersburg,Magnesium sulfate in Novosibirsk,Magnesium sulfate in Yekaterinburg,Magnesium sulfate in Nizhny Novgorod,Magnesium sulfate in Kazan,Magnesium sulfate in Chelyabinsk,Magnesium sulfate in Omsk,Magnesium sulfate in Samara,Magnesium sulfate in Rostov-on-Don,Magnesium sulfate in Ufa,Magnesium sulfate in Krasnoyarsk,Magnesium sulfate in Perm,Magnesium sulfate in Volgograd,Magnesium sulfate in Voronezh,Magnesium sulfate in Krasnodar,Magnesium sulfate in Saratov,Magnesium sulfate in TyumenMode of application
Dosage
Doses are specified taking into account the therapeutic effect and the content of magnesium ions in the blood serum.Preeclampsia and eclampsia. Saturation dose - 2-4 g every 5-20 minutes (infusion). Maintenance dose - 1-2 g per hour.
Uterine tetany. Saturation dose - 4 g every 20 minutes (infusion). Maintenance dose - first 1-2 g per hour, later 1 g per hour (can be administered drip-wise for 24-72 hours).
Hypomagiemia.
In newborns. The daily dose is 0.2-0.8 ml/kg slowly intravenously.
In adults. Easy. A solution of magnesium sulfate is used parenterally if it is impossible to take magnesium preparations orally (due to nausea, vomiting, impaired resorption in the stomach, etc.) the daily dose is 1-2 g. This dose is administered once or in 2-3 doses.
Heavy. The initial dose is 5 g, which is delivered in 1 liter of infusion solution and administered slowly intravenously. Dosage depends on the concentration of the drug in the blood serum.
Prevention of hypomagicemia in patients receiving only parenteral nutrition. If there is no magnesium in the nutrient solutions, it is added additionally. The daily dose is 1.5-4 g. Typically, 1 g of magnesium sulfate is added to 1 liter of parenteral nutrition solution. The maximum daily dose of magnesium sulfate for adults is 40 g.
For hypertensive crises, 5-20 ml of magnesium sulfate solution 250 mg/ml is administered intravenously (slowly).
To relieve arrhythmias, 1-2 g are administered intravenously over about 5 minutes, repeated administration is possible.
Doses of magnesium sulfate are indicated in grams. They correspond to the amount of solution 250 mg/ml: 1 g - 4 ml; 2 g - 8 ml; 3 g - 12 ml; 4 g - 16 ml; 5 g - 20 ml; 10 g - 40 ml; 15 g - 60 ml; 20 g - 80 ml; 30 g - 120 ml; 40 g - 160 ml. Magnesium sulfate solution in ampoules is diluted injection solutions: 0.9% sodium chloride or 5% dextrose (glucose).
Procedure for working with a polymer ampoule:
1. Take the ampoule and shake it, holding it by the neck.
2. Squeeze the ampoule with your hand, without releasing the drug, and use a rotating motion to turn and separate the valve.
3. Immediately connect the syringe to the ampoule through the resulting hole.
4. Turn the ampoule over and slowly draw the contents into the syringe.
5. Place the needle on the syringe.
Overdose
Symptoms:Disappearance of the knee reflex, nausea, vomiting, sharp decrease in blood pressure, bradycardia, respiratory depression and central nervous system depression.
Treatment:
A 10% solution of calcium chloride or calcium gluconate is slowly injected intravenously - 5-10 ml, oxygen therapy, carbogen inhalation, artificial respiration, peritoneal dialysis or hemodialysis, and symptomatic therapy are carried out.